Broadcom MASTERS finalists discuss disparities in healthcare with JASON learning

When faced with a challenge, the 2021 top 30 Broadcom MASTERS finalists knew just how to solve it, especially when working together. This year, JASON Learning and Broadcom MASTERS, teamed up to host a virtual challenge during competition week that asked finalists: “How do pharmaceutical companies overcome barriers to increase diversity and participation in clinical drug trials?” Ensuring that clinical trials are diverse, by including women and racial and ethnic minorities, healthcare data and solutions will be more complete and will help advance health outcomes for all. Diversity in clinical trials is one of the keys to advancing health equity, as it better represents the patient populations who will benefit from forthcoming medical products, therapies and treatments, improving overall trust in medicine.
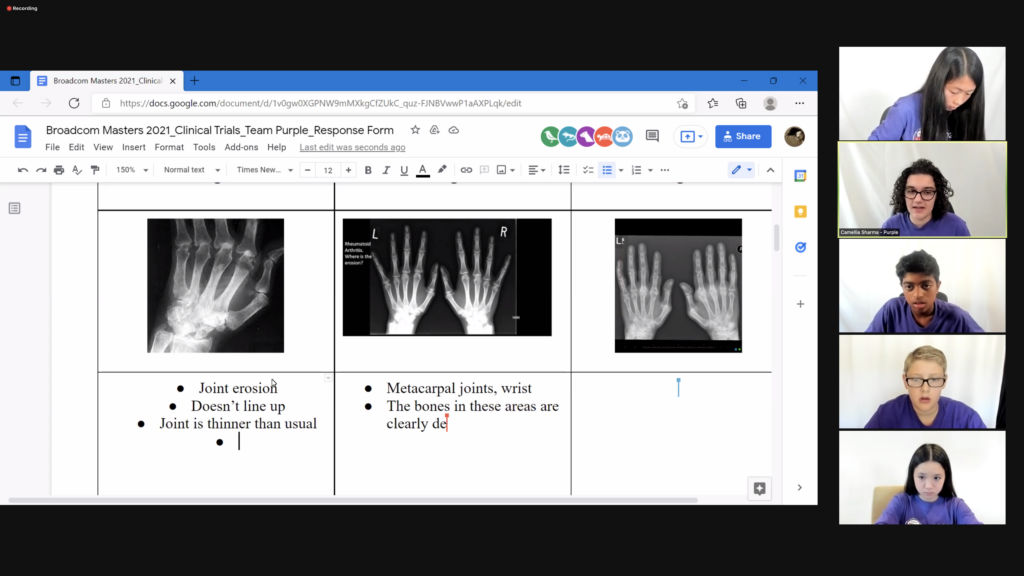
The finalists broke into six groups and reviewed x-rays of the hands of patients showing signs of rheumatoid arthritis. Each group analyzed the scans and noted symptoms of the disease, observing signs of inflammation, decreased bone density, joint misalignment and a lack of space between bones. Each team then brainstormed how participation in a clinical trial for a new rheumatoid arthritis drug could be improved.
All six finalist groups identified some of the detrimental impacts resulting from existing health inequities in current clinical trials and the healthcare system as a whole. They discussed eligibility requirements to participate in trials and proposed solutions to inequities. For instance, the gold team discussed the difference between obstacles and reluctance toward participation in trials and considered how disabilities could impact eligibility. The black team considered where eligible participants get their information from. Are they finding information about participation via social media, local and/or national news, and are those modes of communication and news impacting who is participating? The white team discussed how factors like location, language, and cultural and religious beliefs had an impact on participation. The silver, purple and green teams focused on demographic information during their analysis. They each discussed how low participation across demographics could impact both doctor and patient confidence, as well as drug effectiveness.
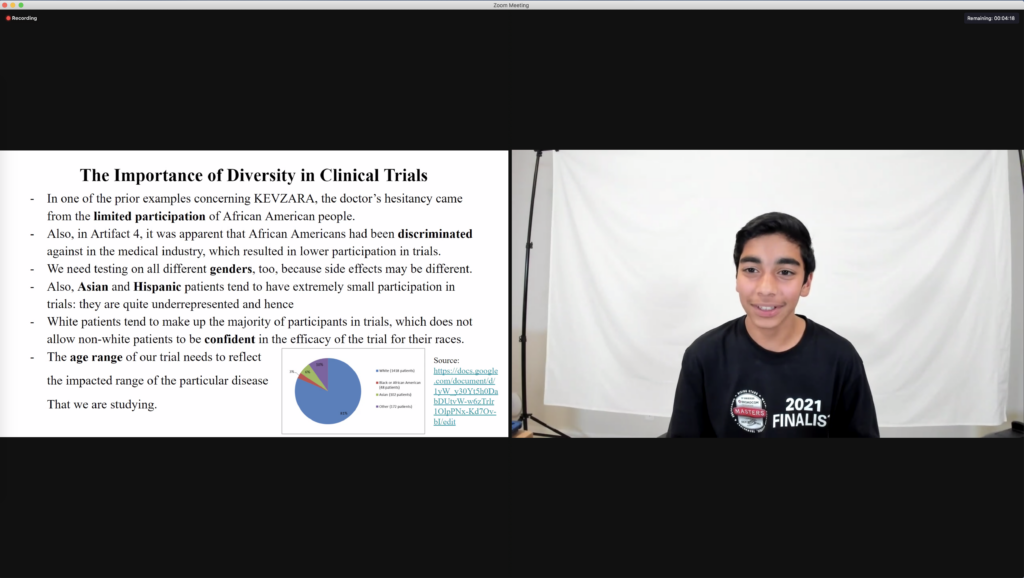
Finalists then worked together to create their own clinical trial model, allowing them to critically think about data from previous trials and note demographics that needed more representation. The groups collaborated to consider how providing resources and support for patients during the trials could improve participation. All teams agreed that demographic diversity needed to be increased to improve the trial drug’s efficacy. Eligibility requirements such as age range, race and gender were also determined to be necessary. For their own clinical trial models, each team considered compensation for trial participation, along with patients’ legal rights in the case of mistreatment. One team even considered how the location of the clinics could impact patient access to the trial, meaning more clinic locations in underrepresented communities could increase diversity.